Abstract
Introduction: Daratumumab is approved across lines of therapy for multiple myeloma (MM) by subcutaneous administration (DARA SC) or by intravenous administration (DARA IV). In clinical studies, the administration time ranges from 3-5 minutes for DARA SC versus 3-7 hours for DARA IV, and DARA SC is associated with reduced rates of administration-related reactions (ARRs). These characteristics of DARA SC are associated with benefits for clinics in terms of safety and clinic time. We previously reported DARA administration characteristics at Mayo Clinic infusion centers: DARA SC had shorter total clinic and total chair times compared with DARA IV, and DARA SC was associated with very low rates of ARR-related events (Soefje S, et al. EHA Library. 2021). Based upon this analysis, the treatment plan was modified to reduce the mandated observation time for DARA SC to improve clinic efficiency. Here, we describe updated clinical administration characteristics for DARA SC at Mayo Clinic infusion centers before and after the reduction in procedure-mandated observation time, versus DARA IV, using a novel empirical data extraction approach from Electronic Health Records (EHR).
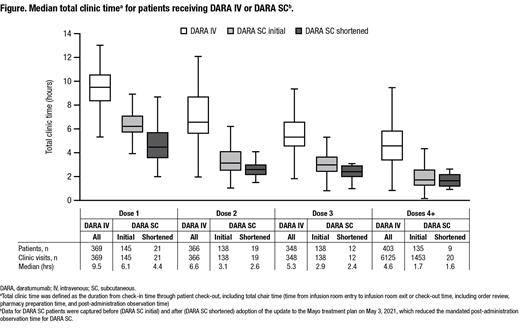
Methods: Patients ≥18 years of age with an ICD-9/10 diagnosis code of MM and a first DARA treatment between April 5, 2017 and June 22, 2021 were identified in Mayo Clinic's EHR database. Data were extracted using a scheduling and pharmacy software that tracked patient movement through appointments at a Mayo Clinic infusion center. On May 3, 2021, the Mayo treatment plan was amended to shorten the mandated post-administration observation time for DARA SC from 4 to 2 hours for Dose 1 and from 1 hour to 30 minutes for Doses 2 and 3, with no mandated observation time for Doses 4+. In this analysis, data were captured for patients initiating DARA IV throughout the defined treatment window. For DARA SC, data were captured for patients starting DARA SC therapy on the initial treatment plan (before May 3, 2021; DARA SC initial) and after the change to shorten the mandated post-administration observation time for DARA SC (after May 3, 2021; DARA SC shortened). Time-based measures included: total clinic time (check-in time through patient check-out); total chair time (time from infusion room entry to infusion room exit or check-out time, including order review, pharmacy preparation time, and post-administration observation time); and observation time (time from the end of medication administration to infusion room exit or patient check-out). The medication administration time documented for DARA SC was 0 minutes due to the default duration for SC injections in the EHR.
Results: In total, 755 (DARA IV, n=586; DARA SC initial, n=145; DARA SC shortened, n=24) patients received DARA treatment for MM. For all doses combined, the median total clinic time was 2.9 hours shorter for DARA SC compared with DARA IV (DARA IV, 4.9 hrs; DARA SC initial, 2.0 hrs). The median total clinic time for DARA SC and DARA IV was highest at Dose 1 (DARA IV, 9.5 hrs; DARA SC initial, 6.1 hrs; DARA SC shortened, 4.4 hrs) and lower for subsequent doses (Dose 4+: DARA IV, 4.6 hrs; DARA SC initial, 1.7 hrs; DARA SC shortened, 1.6 hrs; Figure). Similarly, the median total chair time was 2.7 hours shorter in the DARA SC group compared with the DARA IV group for all doses combined (DARA IV, 4.1 hrs; DARA SC initial, 1.4 hrs). The median total chair time for DARA SC and DARA IV was highest at Dose 1 (DARA IV, 8.8 hrs; DARA SC initial, 5.6 hrs; DARA SC shortened, 3.9) and lower for subsequent doses (Dose 4+: DARA IV, 3.7 hrs; DARA SC initial, 1.1 hrs; DARA SC shortened, 1.1 hrs).
Conclusion: Marked reductions in the amount of time spent both in clinic and in chair were observed with DARA SC compared with DARA IV, with additional time savings observed following the Mayo procedure change to reduce the DARA SC mandated observation time. These favorable DARA SC administration characteristics may indicate a reduction in the burden on both clinical resources and patients in Mayo Clinic infusion centers. These results add to the growing body of evidence supporting the use of DARA SC as an efficient and convenient treatment administration option for patients with MM.
Soefje: Beigene: Consultancy; Pfizer: Speakers Bureau; Janssen: Consultancy, Research Funding. Carpenter: nference Inc.: Current Employment; Janssen: Consultancy. Carlson: nference Inc.: Current Employment; Janssen: Consultancy. Awasthi: nference Inc.: Current Employment, Current holder of individual stocks in a privately-held company; Janssen: Consultancy. Lin: Janssen: Current Employment. Kaila: Janssen Scientific Affairs, LLC: Current Employment. Kayal: nference Inc.: Current Employment; Janssen: Consultancy. Kirkup: nference Inc.: Current holder of individual stocks in a privately-held company, Ended employment in the past 24 months; Path AI: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Wagner: nference Inc.: Current Employment; Janssen: Consultancy. Gray: Janssen Scientific Affairs, LLC: Current Employment, Current holder of individual stocks in a privately-held company. Kumar: Oncopeptides: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Carsgen: Research Funding; Antengene: Consultancy, Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Bluebird Bio: Consultancy; Beigene: Consultancy; Tenebio: Research Funding; Roche-Genentech: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal